
Dr. Lane Baker,
Associate Professor Indiana University
Dr. Baker’s research group at Indiana University consists of analytical and materials chemists broadly interested in electrochemistry, bioanalytical chemistry, new mass spectrometry methods, materials for electrode fabrication and instrumentation development. He has a great group of student coworkers - right now around 10 - from around the world. At Indiana University, the Analytical Division provides the students with pretty rigorous training in measurement science.

Why is your work important and how is it applied in today’s society?
I think what we do is important to society on primarily two fronts. First, we develop new instruments and materials to help us understand the movement of ions in complex systems. This includes biological samples, like cells and tissues, and synthetic samples, like membranes and polymers. Ions are essentially currency for energy in both of these kinds of systems (living and synthetic), and measurement, control and manipulation of ions at really small scales helps us to better understand how things work. The second, possibly more important part of what we do is to train the next generation of scientists. We try hard to develop students who like to be both “thinkers and tinkerers”. Meaning they have to understand the fundamentals of what they are doing (be a thinker) and they have to have the skills to build new tools and reconfigure or repurpose existing technology (be a tinkerer). These are really the two most important traits we can develop, and I would like to think my student coworkers leave well trained in these areas.
How do you collaborate with other research teams involved in Nanotechnogy research and are they world-wide across a wide range of disciplines?
We work with physicists, cell biologists, other chemists and materials scientists. That is one of the great things about nanotechnology, you can apply it almost anywhere to any field. A lot of our collaborations consist of my lab developing a new tool or method and then working with colleague with a really interesting sample.
How does studying electro chemical transports help with battery life and chemical degradation.
Electrochemical transport we have studied, in this case across a Nafion® membrane is one of the key steps in balancing charge between two halves of a significant number of really important next generation energy technologies (eg fuel cells). When the membrane loses the ability to selectively transport protons (H+), the overall operation can be compromised. With the Park XE-Bio and SECM-SICM probes we developed, we were able to observe the heterogeneity in membrane degradation. This could be a useful tool to help develop next generation membranes that have improved chemical durability.
 This image shows a Nafion© membrane that has been chemically degraded. With SICM-SECM, changes in ion transport through the degraded membrane were observed.
This image shows a Nafion© membrane that has been chemically degraded. With SICM-SECM, changes in ion transport through the degraded membrane were observed.
Why is it important to understand how lithium affects surface charge?
Lithium is the key player in lithium ion batteries, which can be found everywhere. But lithium ions, especially in non-aqueous solvents, which are commonly used in energy applications, can behave in unexpected ways. With our collaborators at the University of California-Irvine (Zuzanna Siwy and students) and Oak Ridge National Laboratory (Ivan Vlassiouk) we were able to make some really neat measurements that showed lithium ions adsorb polycarbonate films and form a surface with a net positive charge. This could be really important for nanostructuring battery materials, where the surface area increases will make surface charge an important consideration.
Why did you develop a new SICM using electro spray and how does it perform vs the traditional method?
We developed an electrospray imaging system (we call is Scanning Electrospray Microscopy, or SESM) to open new routes for surface patterning, and possibly for new routes to surface-desorption mass spectrometry techniques. SESM also gives us the chance to understand the electrospray process at really small scales, which is hard to do.
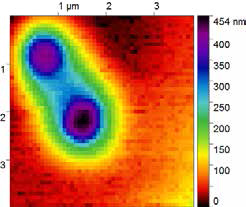 Image collected by Scanning Electrospray Microscopy (SESM) module develop with the Park XE-Bio. Two one micron polystyrene particles can be observed, where electrospray from a nanopipette provided feedback and position control.
Image collected by Scanning Electrospray Microscopy (SESM) module develop with the Park XE-Bio. Two one micron polystyrene particles can be observed, where electrospray from a nanopipette provided feedback and position control.
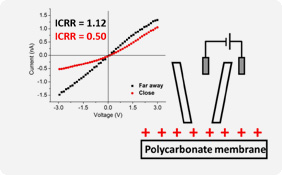 Ion current rectification measurements of surfaces with Park XE-Bio. By measuring the current-voltage rectification response (ICRR) far away from a surface and close to a surface, the net surface charge can be deduced. In plots shown a positive surface charge is measured for a solution of lithium ions in propylene carbonate.
Ion current rectification measurements of surfaces with Park XE-Bio. By measuring the current-voltage rectification response (ICRR) far away from a surface and close to a surface, the net surface charge can be deduced. In plots shown a positive surface charge is measured for a solution of lithium ions in propylene carbonate.
How is SICM helping to measure Ions and how is that different than how it was done in the past?
The big difference SICM makes is the ability to probe small scales, to make local measurements of ion transport. SICM is great because you can take high resolution images in operationally relevant (biological or electrochemical) solutions, and – even more importantly – there are a lot of chemical processes you can measure with SICM or hybrid versions of SICM (like SECM-SICM). In the past ten years the SICM community has really added a lot of exciting measurement ability to SICM.
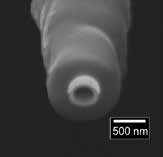 A hybrid scanning electrochemical microscopy – scanning ion conductance microscopy (SECM-SICM) probe. The central barrel is used for SICM and provides distance control, the crescent-shaped gold electrode is used to measure faradaic electron transfer. The entire pipette is insulated in a layer of vapor-deposited parylene.
A hybrid scanning electrochemical microscopy – scanning ion conductance microscopy (SECM-SICM) probe. The central barrel is used for SICM and provides distance control, the crescent-shaped gold electrode is used to measure faradaic electron transfer. The entire pipette is insulated in a layer of vapor-deposited parylene.
How do you use Park SICM for your research and what are the key benefits of Park SICM?
We use the Park for several projects in my research group, especially for our energy related research and for development of scanning electrospray microscopy (SESM). The Park XE-Bio is really easy to use, so most students start out training on it. The Park also has a good physical design and the electronics give us good signal-to-noise. The ability to swap out the SICM head with the AFM head has also proven really useful in several experiments where AFM gives us nice complementary data.
How might we use AFM-based bioanalysis for the study of desease?
AFM is great for measuring a number of cellular properties, especially properties like cell stiffness, which can be very useful for understanding the physiology of disease. One of the more exciting applications of AFM are pull-off based mapping for cell surface receptors, this represents a really exciting, unique application of AFM in helping us understand biology.
Can you describe advances we might see in DNA research using SICM in the future?
SICM has been used recently as a tool to sample DNA from subcellular regions in single cells, DNA which was then used to explore the genomics of the cell. I expect in the future this will prove especially powerful.
You worked with Lloyd Whitman who is now the Asst Director for NanoTechnology at the White House Office of Science and Technology Policty and he has done alot of work on bio sensors. Can you tell us about some of the projects you worked together on and their significance?
I was a NRC postdoctoral associate at the Naval Research Laboratory in Washington DC, and Lloyd was my mentor. In Lloyd’s lab I got to work with a lot of really smart staff scientists (Arnaldo Laracuente, Tom Clark, Paul Sheehan) and other postdocs on biosensors and interfacial chemistry/physics. With Lloyd I got some good training on scanned probe microscopy, specifically scanning tunneling microscopy (STM). I was able to put that training to good use when I started my own research group doing SICM.
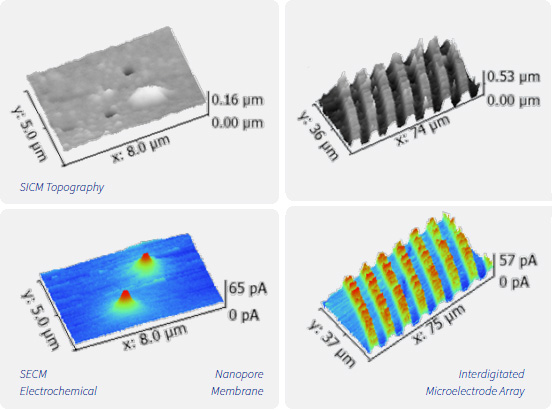 (Top) Scanning ion conductance microscopy (SICM) images of nanopores in a polymer membrane and of an interdigitated microelectrode array.
(Top) Scanning ion conductance microscopy (SICM) images of nanopores in a polymer membrane and of an interdigitated microelectrode array.
(Bottom) Scanning electrochemical microscopy (SECM) is used to meaure diffusion of redox active molecules through the nanopores of the membrane or to measure redox cycling at the microelectrode array.
