 Dr. Harper is an Associate Professor located in the Center for Renewable Carbon at the University of Tennessee. He holds a 100% research appointment in the Tennessee Agriculture Experiment Station. He has been a member of the Forestry, Wildlife, and Fisheries Faculty since 2004.
Dr. Harper is an Associate Professor located in the Center for Renewable Carbon at the University of Tennessee. He holds a 100% research appointment in the Tennessee Agriculture Experiment Station. He has been a member of the Forestry, Wildlife, and Fisheries Faculty since 2004.
Dr. Harper has been a Park XE-100 Scanning Thermal Microscopy (SThM) AFM user since 2006. Currently, Dr.Harper is conducting research in the area of improving the compatibility of naturally derived materials with other polymer systems. His goal is to improve the utility of undervalued natural materials to support rural development, to utilize renewable materials in engineering applications, and to promote environmental stewardship.
Q: What is the field of your research?
My area of research involves studying renewable materials derived from plants and composites (wood, fiber and polymer). Key to my research is investigating the properties of these materials at the micro-scale level. Materials derived from plants can be used in applications such as housing, transportation and eventually as a replacement for many fossil and mineral based materials.
Q: Can you tell us about the study you are doing using an AFM?
I use atomic force microscopy (AFM) to study the morphology of cellulose fibers, lignin based materials, and the adhesive penetration into wood cells using SThM (Scanning Thermal Microscopy). We also use it to measure the elastic modulus of nanocellulose fibrils. Most importantly, we use it to characterize the heterogeneity and dispersion of phases of various natural materials at the nanoscale.
Q: What made AFM the best choice for your study?
A nano indentation instrument could have been used for some of the experimental work. However, I chose to use an AFM because it’s easier to use, and it can obtain high spatial resolution images . Also, one of the key features I liked is that it allows me to find the location of interest before scanning an image, which is extremely important when analyzing heterogeneous specimens.
Q: How well is Park AFM meeting your research needs?
(It is) very well so far. I am using a Park XE-100 and it’s meeting most of my research needs.
Q: What are the key features of Park AFM do you like the most?
One of the key features I like is phase imaging, which is very useful in detecting the homogeneity of polymer blends. I also like the non-contact mode available in Park AFM. I use the contact mode to teach students about AFM in general; however, I prefer and use the non-contact mode because it saves the tip from wearing issues, especially if you need to scan a lot. With noncontact, I can maintain a sharp tip which is important for image resolution, and in the end it saves me money.
Q: Which is more important to you? Accuracy vs. resolution, and vs. scan speed?
While high resolution is important, ultimately the accuracy of the data is the most important criteria for my AFM needs. That’s again true when compared to scan speed; accuracy is the most important factor for my research.
About the Research Using Park AFM:
Adhesive penetration of wood cell walls investigated by scanning thermal microscopy
As for studying the adhesives, Dr. Harper et. al realized there was a need to examine woodcomposite properties at the micro-scale to learn more about the cell wall and adhesive polymer interaction. The penetration of the adhesive polymer in wood cell is very important to study because it can change the mechanical properties of the regions affected. This can be very essential for industrial applications and scientific knowledge. While there are many other techniques that can be used such as optical microscopy and X-ray analysis for this type of study, Park’s AFM with the SThM was chosen because of the high spatial resolution that can be obtained with Park. Furthermore, the sample preparation is simple without the need for any chemical modification. In addition, more information can be obtained from the 2D distribution maps delivered by means of SThM in regards to adhesive distribution.
Measurements of SThM were done using two modes on Park AFM, thermal contrast microscopy (TCM) which measures the temperature variations on the surface, and conductivity contrast microscopy (CCM) which measures the conductivity of the surface while maintaining a constant probe temperature. One of the great features here is that the thermal probe is used simultaneously as a thermal sensor and an AFM tip in true non-contact.
The SThM measurements were performed in the interphase of wood adhesive bonds prepared by bonding the wood specimens with two types of adhesives and epoxy resin. The adhesives used were 1) phenol-urethane (PUR) and 2) phenol-resorcinol-formaldehyde (PRF). Figure 1 shows
the topography of wood cells with PRF and PUR bond line. The authors observed different thermal behavior of PRF adhesiveness in cell walls that is associated with the diffusion of liquid from the bond line into the wood cells, unlike epoxy or PUR. 3D plots of the voltage distribution of the samples were obtained across the cell walls of the samples embedded in epoxy and bonded by PRF. As shown from Figure 2, the wood cell wall in contact with the embedding epoxy displays a homogenous distribution of voltage signal while the wood cell in contact with PRF shows a voltage gradient. This confirms PRF penetration into the wood cells.
In conclusion, SThM was a suitable method to study the adhesive polymer interactions in wood cells. The thermal properties found across the cell walls while in contact with PRF adhesive shows the ability of its penetration, which was not found in cells with PUR adhesive or embedded in epoxy. As a result, Park AFM has shown to be a useful method to study thermal properties at the micro-scale with high accuracy as seen from the work presented here.
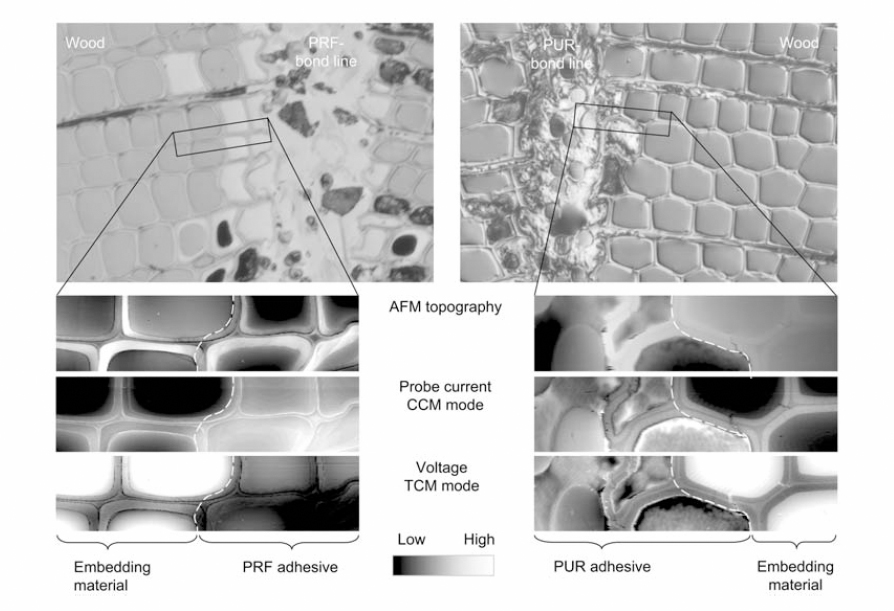
Figure 1 Topography, CCM and TCM Images of wood cells in contact with PRF and PUR
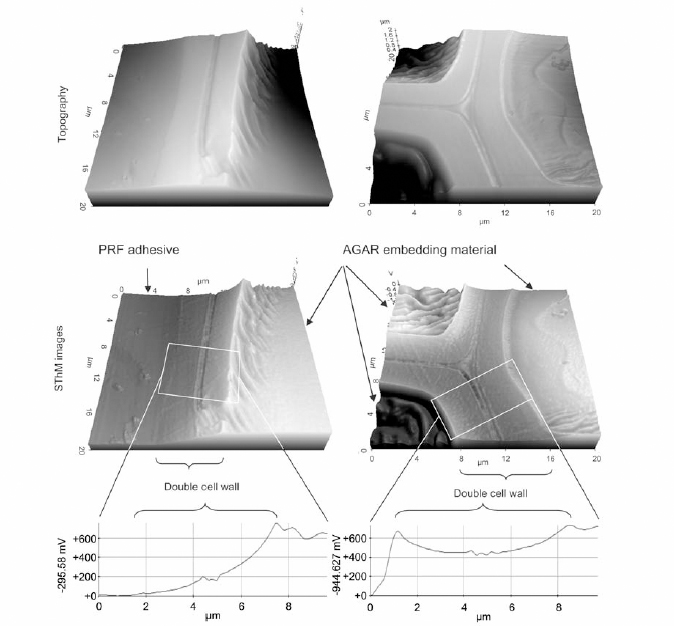
Figure 2 Topography and SThM images of wood cells with and without contact with PRF adhesive embedded in epoxy resin
Published Work Using Park AFM
1) Cheng, S. Wang, D. P. Harper. 2009. Effects of process and source on elastic
modulus of single cellulose fibrils evaluated by atomic force microscopy. Composites Part A.
Accepted.
2) Konnerth, D. P. Harper, S.-H. Lee, T. G. Rials, W. Gindl. 2008. Adhesive penetration of w
ood cell walls investigated by scanning thermal microscopy. Holzforschung. 62(1): 91-98.
