 Dr. Rozlosnik is Associate Professor at DTU Nanotech, Technical University of Denmark. Her research areas include polymer-based biosensors, conductive polymers, cells and viruses, and medical diagnostics. Dr. Noemi is an expert in the areas including microprocessing of polymers, conductive polymers, electrochemical methods, surface analysis by AFM (Atomic Force Microscopy), conductive AFM and force spectroscopy, contact angle goniometry, confocal microscopy, Langmuir-Blodgett thin films techniques, and cell/virus culturing and handling.
Dr. Rozlosnik is Associate Professor at DTU Nanotech, Technical University of Denmark. Her research areas include polymer-based biosensors, conductive polymers, cells and viruses, and medical diagnostics. Dr. Noemi is an expert in the areas including microprocessing of polymers, conductive polymers, electrochemical methods, surface analysis by AFM (Atomic Force Microscopy), conductive AFM and force spectroscopy, contact angle goniometry, confocal microscopy, Langmuir-Blodgett thin films techniques, and cell/virus culturing and handling.
Dr. Rozlosnik is a pioneer user of various AFMs over the span of 20 years. Recently, she has been using Park XE-Bio SICM (Scanning Ion Conductance Microscopy) to conduct her research.
Q: What is the field of your research?
Our main focus is to develop micro-fabricated all-polymer based biosensors, in which we are
using conductive polymer electrodes for electrochemical detection. It is really a new field to make
sensors just from plastic. The basic idea is to produce inexpensive diagnostic devices, which can
also be used in poor countries. Our sensors are made from plastic, which cost maximum 5
dollars or even less, depending on what kind of functionalization we are making on the surface.
We are developing sensors not only for medical diagnostics, but also, for example, detection of
antibiotics in food samples, and for detection of small molecules for environmental studies.
Q: Can you tell us about the biosensor you’ve developed for virus detection?
The sensor microchip for virus detection is a cell-based biosensor. We cultured cells in the microchannels, and infected with the virus, and detected the response of the cells by electrochemical impedance spectroscopy. And we investigated the infection process with AFM and SICM.
The AFM, and the SICM are really useful tools for characterizing cells in the physiological conditions. We could for example image how the viruses are attaching to the surface, and we could correlate the impedance changes in the biosensors to this effect. (See in the paper titled “Polymer Based
Biosensor for Rapid Electrochemical Detection of Virus Infection of Human Cells”). If you look the cells with an SICM, you see the surface changes from the attachment and also some tiny structures on the surface in high resolution.
Q: Is Park SICM enabling you to do new things that were not possible before?
Yes, mainly on biological samples. The most important is that you can see more detailed information on the surface because the SICM is not destroying the surface of the cell. Even though True Non-Contact [the unique feature on all Park AFMs] is a good method for imaging cells, SICM gives better imaging cell in biology. Another application is to study conductive polymers, where we can study conductivity via ion current, which is really interesting. Most conductive polymers are conducting both with electrons and with ions. Without SICM, it would be impossible to study these. With STM, you are just detecting electron-tunneling current, not the ion current. So I think SICM is a really new application.
Q: How easy was it to use Park SICM?
I have spent about 20 years with AFMs, and have used at least 5 different types of AFM in my life, so that means for me it was not a big step to learn. However, the [Park SICM] software is pretty good. If you understand the idea behind SICM, then it’s easy to use.
Q: How about the use of micropipettes on Park SICM?
I was using both glass and quartz. The quartz was a little bit tricky because it stopped working after a short time, so it was not as good as the glass, but for the high resolution SICM I used a quartz capillary. You should really take care of not touching the surface, but on the other hand it gives better resolution.
Q: Any comments you would like to share?
I would like to say that SICM has a big potential in the ion current measurements. It’s really important for cells. I have already seen papers about measuring ion current directly through the ion channels in the cell membrane, which, of course, is not possible with other methods
Q: How well is Park AFM meeting your research needs?
I have used many different AFMs, but always preferred the True Non-Contact method [of Park AFM]. The True Non-Contact method gives better results especially in soft samples. I got a couple of nice results with a very high resolution.
[About Park Systems]
I have been in contact with the company for many, many years, and I never had any problem with support, which is very good. If I had any problem with the instrument, I’ve got help very fast. I’m fully satisfied with the company [Park Systems].
About the Research Using Park AFM:
Polymer Based Biosensor for Rapid Electrochemical Detection of Virus Infection of Human Cells
Abstract. The demand in the field of medical diagnostics for simple, cost efficient and disposable devices is growing. Here, we present a label free, all-polymer electrochemical biosensor for detection of acute viral disease. The dynamics of a viral infection in human cell culture was investigated in a micro fluidic system on conductive polymer PEDOT:TsO microelectrodes by electrochemical impedance spectroscopy and video time lapse microscopy. Employing this sensitive, real time electrochemical technique, we could measure the immediate cell response to cytomegalovirus, and detect an infection within 3 hours, which is several hours before the cytopathic effect is apparent with conventional imaging techniques. Atomic force
microscopy and scanning ion conductance microscopy imaging consolidate the electrochemical measurements by demonstrating early virus induced changes in cell morphology of apparent programmed cell death.
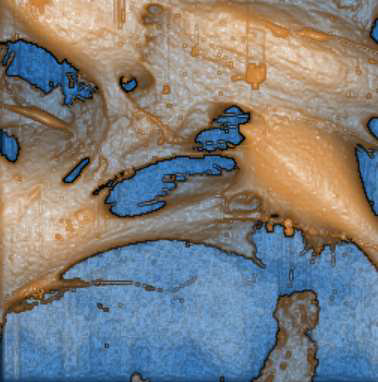
Polymer based biosensor for rapid electrochemical detection of virus infection of human cells
Kiilerich-Pedersen, Katrine ; Poulsen, Claus R. ; Jain, Titoo ; Rozlosnik, Noemi
in journal: Biosensors and Bioelectronics (ISSN: 09565663) (DOI: http://dx.doi.org/10.1016/j.bios.2011.07.053), vol: 28, issue: 1, pages:
386-392, 2011
Type: Journal article - Journal article
DOI: 10.1016/j.bios.2011.07.053
