Lipid Vesicle and Bilayer
AFM Imaging as a Tool to Visualize Bilayer Formation from Lipid Vesicles
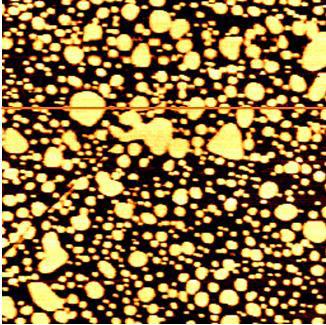
Figure 1. AFM image of lipid vesicles and bilayer in liquid imaged with the XE-100 (3 μm scan size).
Phospholipid bilayers are important in biology because they form the basic structure of the plasma membranes of all living cells. The plasma membrane surrounding the living cell serves several functions, such as control of solute permeability and recognition events.
AFM is an ideal tool for studying both physical and chemical characteristics of those membranes at a highly localized level since it can uniquely generate high resolution images in liquid.
Lipid bilayer is usually deposited onto the substrate using so-called vesicle fusion technique. Figure 1 shows a topographic image of partially flattened vesicles and lipid bilayers. The image was obtained using contact mode AFM in buffer solution. When the vesicles hit a suitable solid surface, they may adsorb, break up, and spread to form a bilayer on a hydrophilic surface. The fused vesicles produce flat domains as shown in Figure 2, 3 and eventually will cover more of a solid surface.
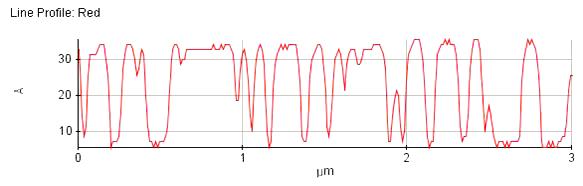
Figure 2. Line profile of lipid vesicles and bilayer on mica surface 3 μm scan size).
The sequential vesicle fusion process such as adsorption, rupture and spreading can be studied at each stage using AFM. Besides dynamic processes, AFM can also be used to image lipid-protein mixed phospholipid bilayer for further studies. It is also possible to separate different kinds of phospholipids mixture into distinct domains since there is a difference in height or structure between the species.
Due to its excellent resolution, the Park AFM is useful for fundamental studies of lipid membranes such as dynamics, structure, and biomolecular interactions.
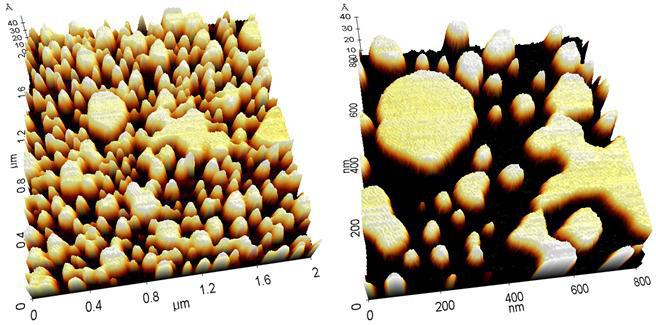
Figure 3. 3D rendering of AFM images of lipid vesicles and bilayer in liquid with the XE-100 (2 μm, 0.8 μm scan size).
